Abstract
Children with Down syndrome are at elevated risk (10-20-fold) for the development of leukemia. The DS-myeloid leukemia (DS-ML) treatment relies largely on low-dose cytarabine (Ara-C). Despite the favorable outcome, there is 20% mortality rate associated with DS-ML. There is no targeted therapy so far for the patients who fail to respond to Ara-C treatment. Majority of clinical trials exploring investigational drugs for AML exclude DS patients because of differential drug sensitivity. Patient-derived xenograft models of DS-ML are limited, because of low sample availability and low success of engraftment. Therefore, an ex vivo drug testing model for DS-ML that can avoid the pitfalls of 2D culture system is a powerful tool to test novel therapies and understand the drug response mechanisms. To overcome the shortfalls of the previous in vitro drug testing systems for AML, we developed a 3D model that facilitates efficient interaction between leukemia cells with bone marrow mesenchymal stem cells (BM-MSCs) in a 3D microenvironment mimicking the extracellular matrix (ECM) components and modulus of BM.
We generated pediatric BM-MSC lines from donor BM aspirates by their ability to adhere to tissue culture plastic. The immunophenotype analysis revealed that BM-MSC lack the expression of CD34 and CD45 but express CD90, CD44, CD105, CD73, CD166 and CD146. MSCs retained the potential of differentiation into the osteogenic, chondrogenic and adipogenic lineages. In addition to the cellular component, the BM microenvironment consists of various ECM proteins that contribute to the mechanics of the BM, and have profound effect on cellular phenotype and metabolism. PEG provides a blank slate for analyzing the peptide specific cellular responses, and can be further tuned to specify 3D architecture. The hydrogel-based 3D gel system that we developed for the generation of 3D model for transient abnormal myelopoiesis (Sidhu et al., Biomater Sci, 9:6266, 2021) was further tuned by the incorporation of BM mimetic peptides with composition and ratio determined by a proteomics study using human BM (Jansen et al., bioRxiv, 2018).
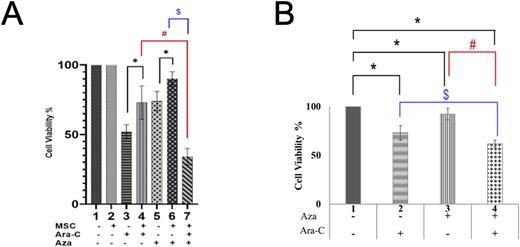
To develop a BM model for drug testing, PEG gels containing BM-ECM mimicking peptides were used to encapsulate CMK cells alone and in co-culture with BM-MSCs. CMK cells treated with 0.5µM of Ara-C showed higher viability in co-culture (73% ± 12%) when compared to monoculture (52% ± 5%) (Figure 1A; bars 3 & 4; p*= 0.012). Similar to Ara-C, treatment with 0.75µM Azacitidine (Aza, a DNA hypomethylating agent) had a significantly higher viability in co-culture (90% ± 5%) compared to monoculture (74% ± 7%) (Figure 1A; bars 5 & 6; p*= 0.031). The chemoprotection of BM-MSCs against individual drug (Ara-C and Aza) was overridden by the sequential therapy with 0.75µM Aza for 24h, followed by 48h treatment with 0.5µM Ara-C under BM mimetic conditions. Compared to Ara-C alone treatment, cell viability was significantly lower in sequential therapy, with 39% (Figure 1A; bars 4 & 7; p#< 0.01) lower cell viability. Similarly, the sequential therapy reduced the cell viability by 56% when compared to Aza alone treatment (Figure 1C; Bars 6 and 7; p$< 0.01). This implies that Aza treatment can sensitize DS-ML cells to Ara-C treatment to disrupt the chemoprotective environment provided by BM-MSCs. In vitro megakaryocytic DS-ML cells harboring GATA1 and STAG2 mutations (T21-G1-S40) showing DS-ML characteristics were generated as described previously (Barwe et al., Cells, 11:628, 2022). The effect of Aza sensitization was tested in megakaryocytic lineage expanded T21-G1-S40 cells. Similar to CMK, the sequential treatment of T21-G1-S40 megakaryocytic cells show reduced cell viability (62% ± 6%; Figure 1B, bar 4) than the individual treatment with Ara-C (73% ± 7%; Figure 1B, bar2) and Aza (92% ± 6%; Figure 1B, bar 3).
In summary, we established a synthetic 3D BM mimetic ex-vivo model consisting of the ECM components, mechanics and one of the key cellular components (MSCs) of BM microenvironment. The pediatric BM-MSCs induce chemoprotection to cytotoxic drugs in DS-ML cells. This chemoprotection can be overridden by the use of epigenetic modifier azacitidine. This study provides proof of principle for establishing a BM mimicking ex vivo model that can be utilized for high throughput drug screening.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal